Hillcrest Medical Center Is First To Use Newly Approved Heart Treatment Device
A device used for the first time in the world by cardiologists at Hillcrest Medical Center's Oklahoma Heart Institute received authorization by the FDA.Friday, October 7th 2022, 6:16 pm
TULSA, Okla. -
A device used for the first time in the world by cardiologists at Hillcrest Medical Center's Oklahoma Heart Institute received authorization by the FDA.
"Our goal at Oklahoma Heart really is to bring new devices and technologies to Tulsa and Northeast Oklahoma and the surrounding communities. So patients don't have to suffer or they don't have to undergo a risky open heart surgery or travel to another city like Dallas or Kansas City or Chicago to get treatment and a research study. Because we have those same studies that they have in big centers and big cities right here in Tulsa," said Dr. Kamran Muhammad, Cardiologist.
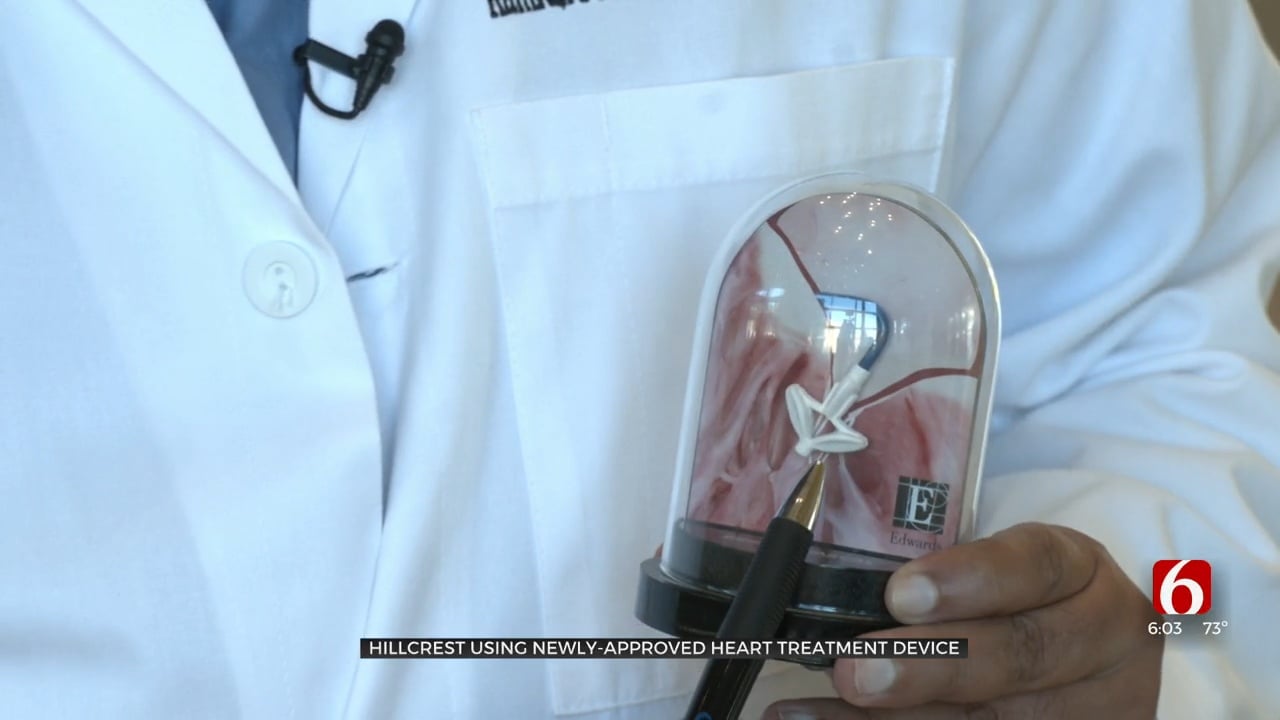
The Edwards PASCAL Precision System is used to treat patients at high risk for surgery for severe cases where the heart valve won't close fully allowing blood to flow back into the heart.
"In the area of cardiology, we often deal with diseases of the heart valves and the heart valves are very important because they open and close every time your heart squeezes and they allow the blood to flow in an orderly direction out of your heart," said Dr. Muhammad.
Life looked a lot different two years ago for Bennetta Yaeger, who has valvular heart problem.
"I could probably walk from here to that chair and sit down to rest. I was tired," said Bennetta Yaeger, Patient. "That's not living. Sitting in a chair is not where it's at."
Yaeger said Dr. Muhammad gave her, her life back. It all started with a device called PASCAL Precision designed to repair a patient's mitral valve by lessening or even stopping it from leaking.
"When you have valvular heart disease or a problem with one of your valves, it can either be that the valve doesn't open well or the valve leaks. Here we were dealing with an issue where the mitral valve was leaking. We call that mitral-regurgitation and when that happens the blood flows backwards in a patient's heart every time the heart beats," said Dr. Muhammad.
"I went into his office for an office call, wanting help, not knowing I would be the first patient," said Yaeger. "It became only the second device available in the united states to treat this condition."
Dr. Muhammad said the Oklahoma Heart Institute was one of two Oklahoma hospitals and the only one in Tulsa participating in this major study.
Hillcrest Medical Center said the FDA approval was based on results from the CLASP IID/IIF Pivotal Trial in 2021. The results of the CLASP IID study were presented and published at the TCT conference in Boston in September 2022.
"We were the first implant this device in Oklahoma and actually we were the first in the world to use this particular model of the device PASCAL Precision when it was rolled out to all the study teams," said Dr. Muhammad.
He said the device is a game changer and allows for a quicker recovery process, is much less invasive and comes with fewer risks.
"It's a very mechanical issue which has a mechanical fix," said Dr. Muhammad. "We can go through the groin vein, the femoral vein and implant the device on the valve while the patient's heart is beating without any open-heart surgery and repair the valve."
"Keep on keeping on, Dr. Muhammad," said Yaeger.
Yaeger said following her procedure last spring, she spent two nights in the hospital and a couple of weeks in rehab.
"Here I am. I really feel like a new person, and I'm not young. 84, ha-ha," said Yaeger. "There's a future out there for me."
Dr. Muhammad said PASCAL Precision performed in a similar fashion to the MitraClip, which was approved in 2014. But he told us there are some distinct differences such as the way it is designed, the way it functions and its physical properties.
He said now there is a new device on the market giving them more options for various anatomy.
Dr. Muhammad said it's like having more tools in a toolbox and believes it'll help a lot of people and have a wide reach.
More Like This
April 20th, 2025
April 20th, 2025
April 20th, 2025
Top Headlines
April 22nd, 2025
April 22nd, 2025