Oklahoma Doctors Celebrate Potential Breakthrough In Brain Cancer Treatment
Doctors at the Oklahoma Medical Research Foundation are celebrating what could become a breakthrough in brain cancer treatment. News 9's Karl Torp has the details.Wednesday, September 16th 2020, 5:56 pm
OKLAHOMA CITY -
Doctors at the Oklahoma Medical Research Foundation are celebrating what could become a breakthrough in brain cancer treatment.
It's a drug that could become the only answer for those facing the devastating diagnosis.
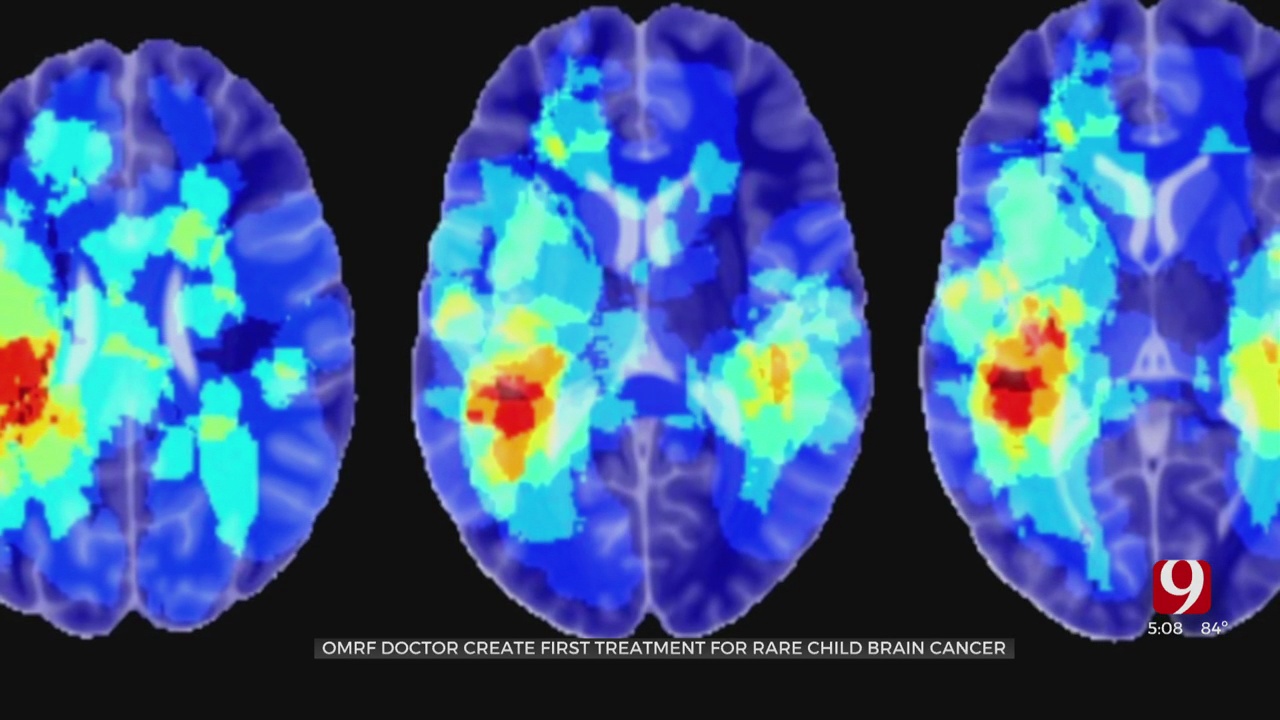
Researcher Dr. Rheal Towner has designed a medicine that fights DIPG, an uncurable form of pediatric brain cancer that effects around 300 children in the U.S. annually.
It took Dr. Towner around 30 years of research to come up with the medicine.
“This drug is actually really good and inhibiting tumor development in what we initially discovered was brain tumors,” said Towner.
The drug is called OKN-007.
It's now been FDA approved for clinical trials in hospitals and is expected to start early next year.
Its primary use was to treat glioblastoma in adults, but results showed promise in younger patients as well.
“We were surprised that it had much more of an affect than we anticipated,” added Towner.
If approved, OKN-007 would become the only FDA treatment for DIPG.
"Our drug is going to provide hope," said Towner.


Karl Torp
Karl Torp is an award-winning journalist who’s been part of the News 9 team since 2012. He co-anchors the 4 p.m., 5 p.m., 6 p.m. and 10 p.m. newscasts on weekdays. Karl loves telling Oklahoma’s unique stories, and he’s also a huge sports junkie. He loves to think of trades that would help the Oklahoma City Thunder win a World Championship (despite knowing little to nothing about salary caps and luxury taxes).
More Like This
September 16th, 2020
June 22nd, 2025
June 22nd, 2025
June 22nd, 2025
Top Headlines
June 22nd, 2025
June 22nd, 2025
June 22nd, 2025